|
|
|
|
|
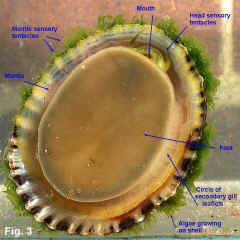
Cellana tramoserica (Holten, 1802) Description: Shell variable in height with apex slightly in front of centre. Sculptured with 30-40 strong radial ribs with narrow interspaces, crossed by dense, fine growth lines; margin finely scalloped. Interior slightly iridescent; margin marked internally with black radial lines showing through from external ribbing. Spatula well defined, varying in colour from grey to white. External coloration very variable; black, yellow, pink or brown, sometimes with ribs reddish or orange. Size: Up to 65 mm in length. Distribution: Endemic to Australia: Burnett Heads, Queensland, to Great Australian Bight, including Tasmania. Habitat: This species lives on exposed rocky shores except for those with the strongest wave action. It occurs from the shallow subtidal almost up to the top of the littoral zone where suitable habitat is available. It lives on rock surfaces which appear bare to the naked eye, consuming the fine film of microalgae from the rock surface. It feeds only when covered by the tide, otherwise remaining motionless. Close examination of the rock surface grazed shows that all algal spores and microalgae are removed except in cracks and crevices (Underwood & Jernakoff, 1981). It is unable to live in areas where macroalgae covers the rocks because it cannot adhere to the substrate or feed on the mature macroalgae. Conversely, its grazing is one of the causes for foliose algae being restricted to low intertidal levels (Underwood, 1980). Sexes are separate, with eggs and sperm being broadcast into the sea. Anderson (1962) studied reproduction in the laboratory, finding that after fertilisation, eggs develop into trochophore larvae after 22 hours and then into veliger larvae on the second day. Settlement occurs on the fourth day, followed by metamorphosis into the adult form Underwood (1974) studied the population of this species at Cape Banks in Botany Bay; he found that spawning occurred from June to September at this site. In the following year (Underwood, 1975) he reported growth rates of the population at Cape Banks. He found that settlement of juveniles occurred over a wider period than June to September, indicating that local larvae were supplemented by those from some other locality. Growth was variable between age groups, but, on average, size increased from 6 to 32 mm in about two and a half years. Sexual maturity is reached at an age of one year, when shells are 24 mm in length (Parry, 1982). Underwood (1977) later reported on a study of movement for this species at Cape Banks in Botany Bay, and Green Point in Broken Bay. He found that some of the limpets returned to the same home site after feeding, while others moved randomly, ending up a small distance, less than half a metre, away from the starting point after feeding each day. Mackay & Underwood (1977) studied the Cape Banks population again, observing 120 limpets over 30 days. They found that homing behaviour was variable. Some limpets continued moving for a few days before returning to their home. Others moved to a new position and began homing from there. Other limpets continued to move about, occasionally homing for 2-3 days and then moving on. They concluded that homing behaviour regulates local density and dispersion to maximise utilisation of the algal food resource. The food of Cellana is the thin film of microalgae that grows on rock surfaces, and sporelings of macroalgae. The microalgal film is more abundant in damp areas, and is markedly seasonal, being greatly reduced in summer. It decreases in abundance with height up the shore (Underwood, 1984). Limpet mortality is greatest during spring and summer, particularly on the upper levels of the shore and if limpet densities are high. Cellana shares the same habitat on rocky shore with other common grazing molluscs, particularly Nerita atramentosa and Bembicium nanum. Underwood (1978) investigated competition between these species at Cape Banks by caging them at higher than normal densities and found that Nerita atramentosa out-competed Cellana for food. Cellana is also in competition for space on the shore with sessile plants and animals. The limpets kill freshly settled barnacles by accidental predation, "bulldozing" or crushing. In the situation of a high larval settlement by barnacles, the number of barnacles may exceed the limpets' ability to keep the area clear, and the barnacles will grow so closely that the limpets cannot feed between them (Underwood et al, 1983). At Cape Banks, Underwood and Jernakoff (1981) considered there were few predators for this species. In the mid intertidal bare rock habitat in which it lives, starfish and octopuses are rare, crabs are not large enough to attack the limpets, and fish are mostly herbivorous species. The molluscs Dicathais orbita and Morula marginalba occasionaly eat Cellana, but not if other prey species are available. However, the limpets have escape responses and are not consumed in large quantities. Parry (1982) investigated the population dynamics of this species at San Remo in central Victoria. He found that there were two main predators, which between them accounted for about half of the mortality; oystercatchers fed frequently on the limpets when exposed by the tide, and wrasses ate smaller shells when immersed. The remaining mortality was caused by starvation during summer and autumn, when the supply of microalgae was low. He found that 48% of adult shells died each year, from either predation or starvation. Cellana tramoserica lives on bare rock surfaces, and when uncovered by the tide is subject to the full heating effect of the sun and drying of the wind. It is able to withstand these conditions provided there is sufficient algal food available. The amphipod Hyale media uses the limpet as a refuge and as a pool of water. Underwood & Verstegen (1988) reported that at Cape Banks, about 10% of limpets had amphipods sheltering under them. Amphipods grouped under some limpets but not others, with up to 26 individuals per limpet. Only limpets that were not in pools sheltered amphipods, and larger limpets were preferred as shelters. The shape of shells varies with position on the shore. Low-profile shells are found subtidally or close to the seaward edge of rock platforms, and high-profile shells live higher up the shore. There are two effects at work here - resistance to waves and resistance to desiccation. Shells on the seaward edge of the rock platform are subject to stronger wave action, and the lower shells with wider base provide less surface area for wave forces. Higher up the shore, desiccation becomes more of a problem, and limpets clamp down more tightly to prevent water loss. This results in the mantle edge, which secretes the shell, being held closer to the body and the shell grows taller (Simpson, 1976). Comparison: Cellana solida (Blainville, 1825) is a similar species that occurs from Wilsons Promontory, Victoria, to Tasmania and SA. Its shell is larger, thicker and stronger than C. tramoserica with fewer ribs, and an orange-red margin internally. Remarks: This is one of the most common shells on NSW rocky shores, and one of the most variable in shape and colouration. The ratio of shell height to length varies from about 0.34 to 0.68 (Fig. 2). The external colouration is very variable, but is usually not seen as most specimens are severely eroded; juvenile specimens tend to be less eroded and show colour pattern and growth lines more clearly. Internal colour pattern reflects the age of the shell; in juveniles the external colour pattern shows through, but the callus that develops as the shell ages makes older shells opaque. Fig. 1 Broulee, NSW (C.075545) Fig. 2 a. Long Reef, Collaroy, NSW (DLB5061) b. Long Reef, Collaroy, NSW (DLB5061) c. Port Phillip Bay, Victoria (C.092831) Fig. 3 Live animal. Long Reef, Collaroy, NSW (C.441411). |